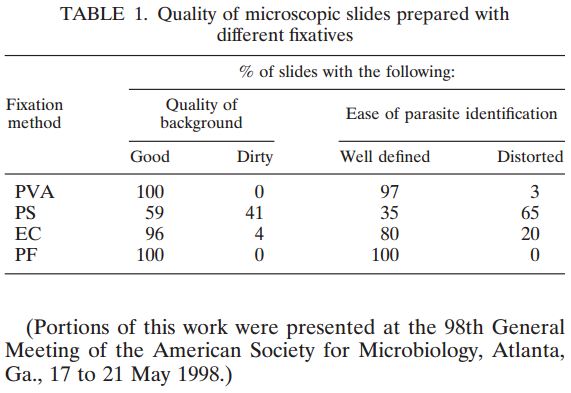
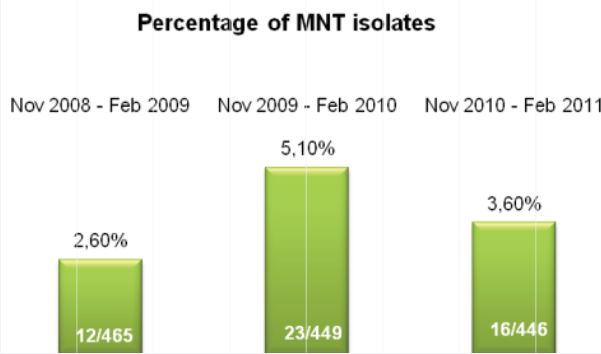
Utilizing quality control slides for staining validation is necessary to ensure reliable, consistent diagnostic results. But making those slides takes time and ensuring accurate, consistent results can be difficult. That’s where Alpha-Tec can help. Whether you specialize in Mycobacteriology, Parasitology, Mycology, and/or Bacteriology, we likely have a QC Slide for you.
Time-Saving Convenience
Regulatory organizations and Good Laboratory Practices dictate that QC Slides be used for specimen processing. However, preparing quality control slides in your laboratory can be a time consuming process and leaves room for potential error. Maintaining cultures or sourcing known positives for everything from Nocardia to Gram can be costly as well.
Alpha-Tec System’s QC Slides save your lab considerable preparation time by eliminating the need to maintain control cultures or utilize positive samples. Our pre-made slides are conveniently packaged in individual pouches to provide accurate validation while only taking moments to set up: just open and stain!
The Importance of Controlling Overgrowth
According to the World Health Organization (WHO), over half of all Tuberculosis (TB) cases globally go undetected each year.2
Sputum smear microscopy remains the most widely used diagnostic; of the 767 million patient evaluations performed worldwide each year, approximately 88 million are sputum smears.3
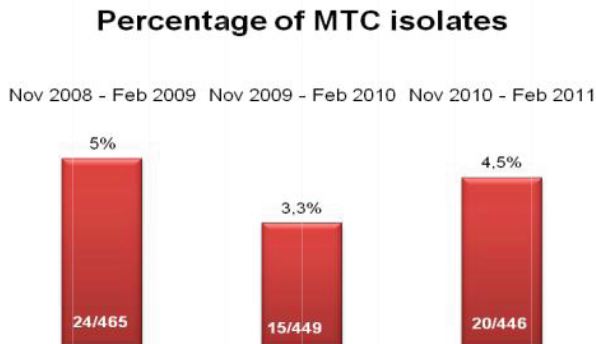
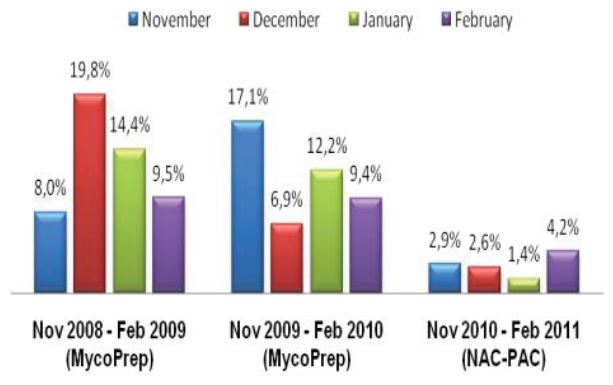
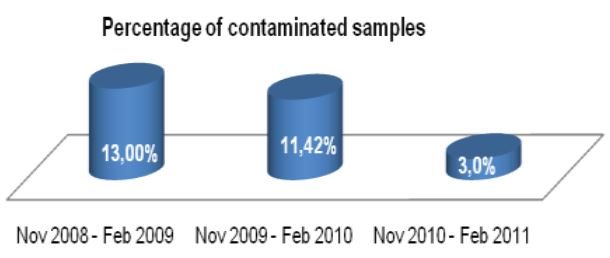
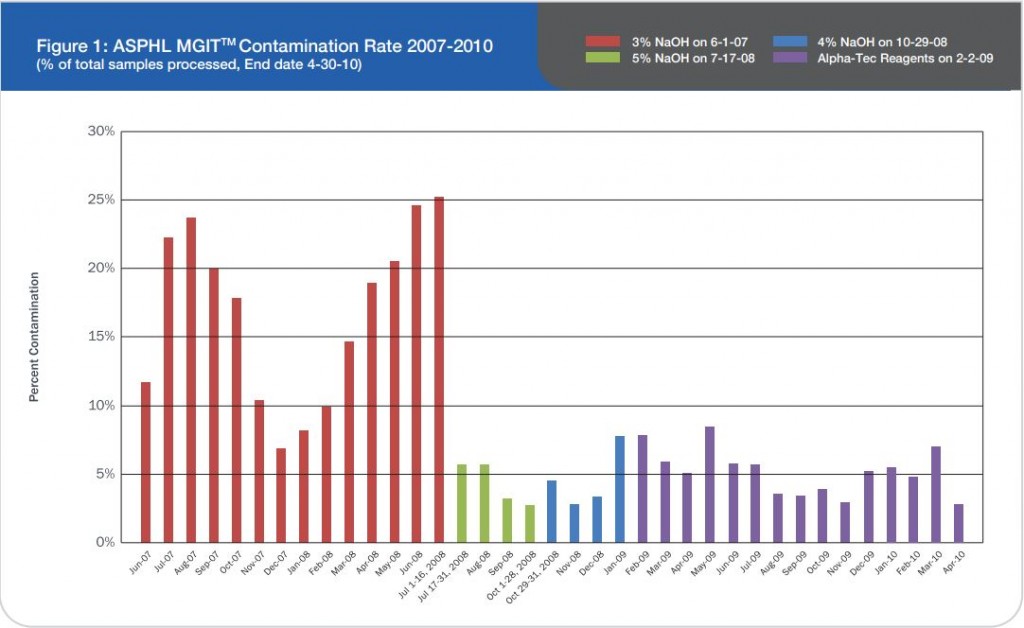
Specimens submitted for mycobacteriology culture frequently contain normal commensal flora in addition to the mycobacteria scientists are hoping to identify. Because mycobacteria are slow growing and require long incubation times, these contaminating non-mycobacterial organisms can result in breakthrough contamination, blocking the ability to detect the presence of the mycobacteria.
Effectiveness of culture systems and microscopy are greatly undermined by contamination with bacteria, fungi and/or commensal flora in sputum specimen samples4, which may result in failure to detect persons with active tuberculosis or cause unnecessary anti-TB treatment for non-TB cases.
In addition, errors in reading sputum microscopy may result in prolonged treatment, or unnecessary treatment termination, which predisposes the development of drug resistant tuberculosis (MDR-TB).5,6,7
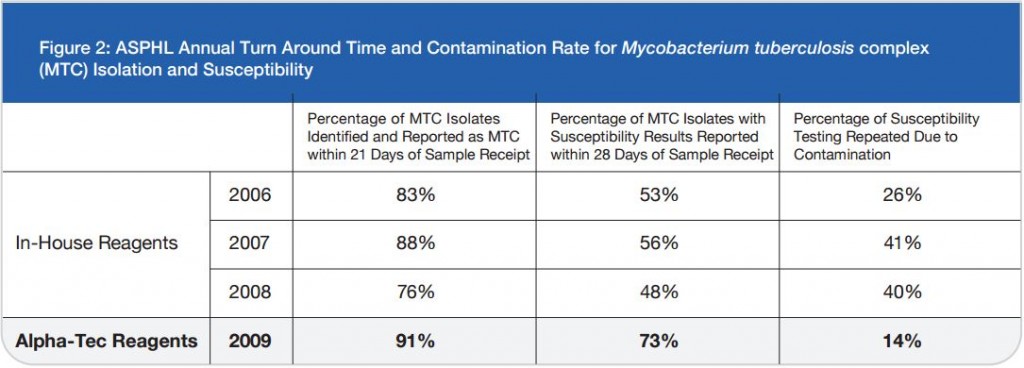
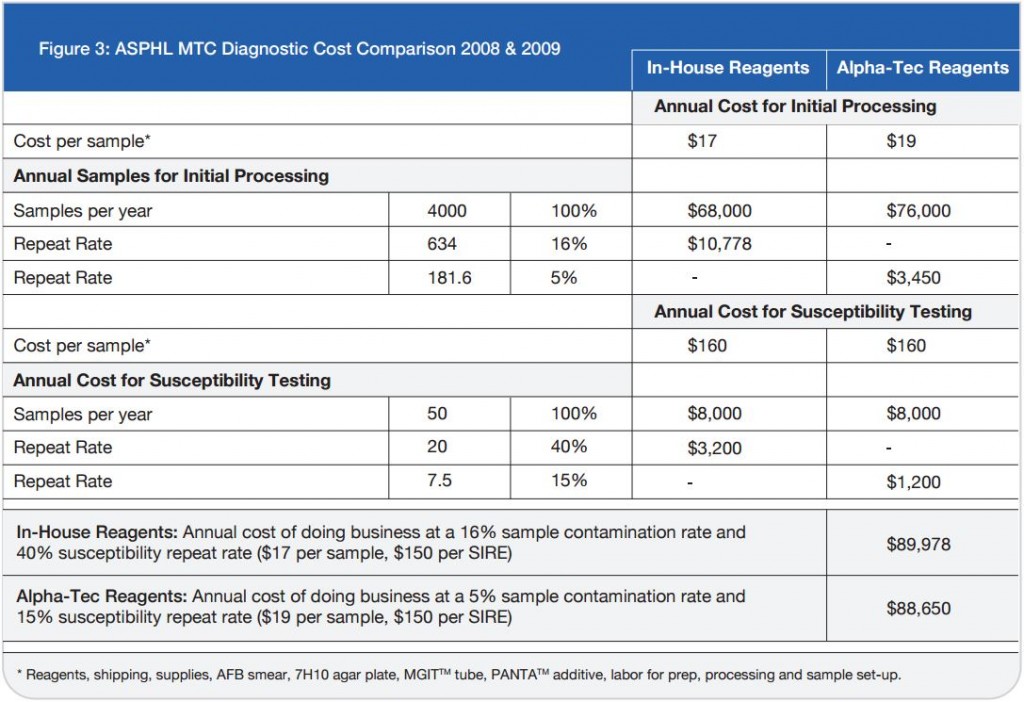
The TRITON2 Sputum Collection System aims to improve quality of sputum specimens reaching laboratories and reduce errors in identifying mycobacteria. By reducing overgrowth in specimens, TRITON2 can aid in a more rapid and accurate TB diagnosis.

Simple Collection
TRITON2 is simple and safe for both patients and care providers to use. The funnel collects specimens directly into a standard 50 ml centrifuge collection tube, preventing contact with the rim of the tube, which then can be tightly sealed, further limiting the possibility of pathogen exposure.
Standardized Processing
With the addition of the buffer and NALC, native commensal oropharyngeal flora overgrowth is inhibited, and mucolytic activity supported, for up to 72 hours. Upon arrival to the lab, the specimen is ready for bacterial studies, molecular processes, staining, or decontamination for AFB culture.
 The TRITON2 Reagent Set (sold separately) is designed as a companion for the TRITON2 Sputum Collection System. It is a pre-aliquoted, open-and-pour decontamination, neutralization, and resuspension kit that standardizes sputum specimen collection and processing. Used together, the TRITON2 Sputum Collection System and Reagent Kit simplify the AFB processing of sputum samples, and provide maximum recovery of viable mycobacteria.
The TRITON2 Reagent Set (sold separately) is designed as a companion for the TRITON2 Sputum Collection System. It is a pre-aliquoted, open-and-pour decontamination, neutralization, and resuspension kit that standardizes sputum specimen collection and processing. Used together, the TRITON2 Sputum Collection System and Reagent Kit simplify the AFB processing of sputum samples, and provide maximum recovery of viable mycobacteria.
Improving Specimen Quality
Pre-liquefied and buffered specimens arrive to the lab ready for bacterial studies, molecular processes, staining, or decontamination for AFB; no need to transfer containers.
Using TRITON2 means less handling, increased specimen quality, and higher confidence in your sputum specimen process.

Specifications:
- Standard 50 ml centrifuge tube
- Stable, removable base
- Safe, ‘no-touch’ re-sealable cap for reduced risk of pathogen exposure
- NALC tablet sealed in oxygen-protected environment within patented cap
- Pre-aliquoted, open-and-pour buffering solution
- Re-sealable transport bag
- Easy-to-follow instructions
- Compatible with: Culture (solid and liquid), PCR/molecular assays, instrumented and manual mycobacterial growth systems, microscopic stains.
| PRODUCT NAME | DESCRIPTION | QTY | PRODUCT # |
|---|---|---|---|
| TRITON2™ | Sputum Collection Device | 20 units | 0005001 |
| 60 units | 0005002 |


Related Links:
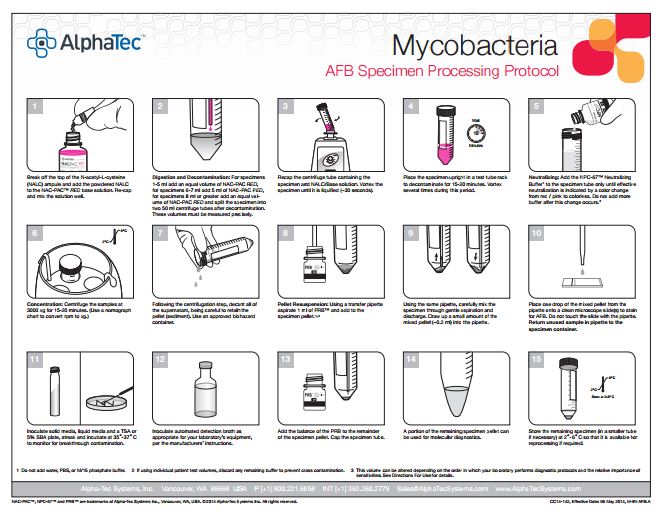
Processing Protocol
Studies
Brochures & Flyers:
Product Flyer (8-1/2×11)(A4)
Customers also purchase: